|
New appointees will enhance the board’s focus on standards, interoperability, and innovation. AIM North America (AIM NA), the trade association for the automated data capture technologies industry (commonly referred to as “AIDC”), including barcode, RFID, IoT, NFC, and RTLS, is pleased to announce the appointment of three additional members to its board of directors, Chris Brown, TSC Printronix; Julie McGill, Trustwell; and Andre Luecht, Zebra Technologies.
Mary Lou Bosco, CEO at AIM North America, emphasized, "Chris, Julie, and Andre bring a wealth of experience and expertise in strategic business development and technology innovation. Their perspectives will be critical as we strive to expand our programs and deepen our impact in the industry."
For more information or photos, contact AIM NA's headquarters by email or call +1.724.742.4473. September 23, 2023, marked the 10-year anniversary of the day the FDA’s Unique Device Identification (UDI) requirement first took effect. For many UDI pioneers and leaders, the date represented a 20-year anniversary since UDI development work began about a decade before UDI became law. In that time, UDI went from an idea to a framework to a law; its GUDID database now uniquely identifies and holds data on more than 4 million medical devices and is the foundation for thousands of daily lookups and transactions. It supports a growing range of use cases in supply chain operations, hospital material management, patient safety and clinical research. UDI set a precedent and serves as a model for similar medical safety and supply chain systems in dozens of countries around the world. The FDA reported that 89 percent of device recall submissions in Q3 2023 included the UDI, double the level from Q1 2022. UDI’s progress is exceptional, considering where it started, and the many diverse stakeholders involved. Yet many professionals that were most responsible for creating and advancing the UDI system are not celebrating these accomplishments but instead are focused on moving the program forward. “Over the last 10 years, a lot of progress has been made,” says Indira Konduri, who is deputy Director of Division of Surveillance Support within the FDA’s Center for Devices and Radiological Health (CDRH), and is leading UDI implementation for the Center. “The good news is that the infrastructure the UDI system needs is in place. Now we’re doing a lot of exciting work leveraging this infrastructure and utilizing UDI to meet the intent of the UDI rule.” AIM NA has supported the UDI program at every step, starting years before UDI became a standard and a regulation. The association provided expert input to every FDA request for comment while the regulations were being developed. Post implementation, AIM provided vendor-neutral technical assistance to help respond to queries the FDA’s UDI help desk. The AIM NA UDI for Medical Devices Work Group remains active in developing resources and sharing knowledge to advance the UDI program and encourages new participants to join. This article presents a snapshot of the current state of the UDI and how the movement may evolve. The Origin Story In the early 2000s, consumer goods were routinely identified with a U.P.C./EAN (now GTIN) number and bar code, and U.S. drug products had the National Drug Code (NDC). There was no standardized equivalent for medical devices. Device makers, safety advocates, regulators and others envisioned a program for medical devices that would go beyond those efforts, which identify items at the category or packaging level, by providing a shareable, unique identification record for every individual device. “The basic reason for the UDI program was to create an NDC system for devices,” says Jay Crowley, who was the original FDA official for the UDI program and now works in the private sector as a UDI consultant and advocate for USDM Life Sciences. He is also the current chair of the AIM NA UDI for Medical Devices Work Group. After extensive consultation among organizations representing various sectors of the life sciences and healthcare sectors, the FDA set the tenets for building the UDI system:
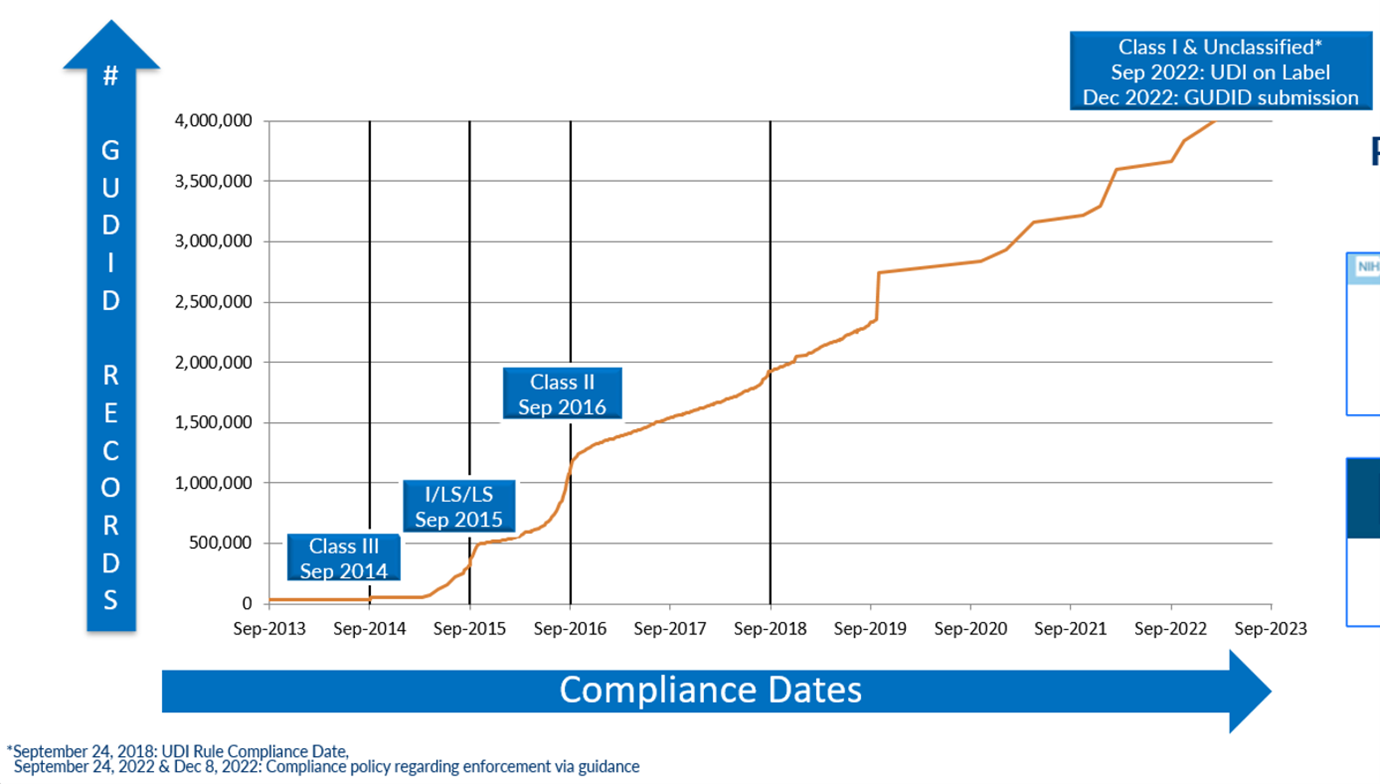
“This new form of device identification, when adopted in health IT systems, could be used as authoritative master data,” says Crowley. “It would increase supply chain efficiency, allow for improved reporting of adverse events, better detection of device safety signals, improved maintenance of biomedical equipment, improved tracking of implants, more efficient and effective recalls, and better monitoring of patient outcomes in clinical care and registry data by replacing unstructured and nonstandard device identification data with a unique number and associated public regulatory data.” To get there, UDI planners had to ask basic questions, starting with, what should be identified? What options should be available for scannable data – could existing standards be used, or was a new one needed? Which database fields should be mandatory and what could be optional? There were hundreds of more detailed questions. Setting the specifications for data formats was especially challenging. A few of the original questions have not been completely answered even as the GUDID database has surpassed 4 million records. There is still some confusion among device makers and others about the minutiae of data formats and their application. Plus, after 10 years, many organizations have lost valuable institutional knowledge because of employee turnover. The biggest remaining question, and frustration, is how to get more organizations using UDI. “We anticipated that moving from ‘chaos’ and nonstandard device to structured identification would take a long time and that, over time, we would uncover and resolve data quality issues,” says Terrie Reed. She is a former FDA official who now helps healthcare organizations take advantage of UDI in their operations in her role as chief strategy officer at Symmetric Health Solutions. “We did not anticipate that each manufacturer would interpret the regulation and its intention differently, and that so much emphasis would be placed on meeting FDA regulatory requirements vs. working with healthcare systems to understand how to implement UDI in a way that would achieve expected patient safety, device safety, supply chain and other commercial benefits.” “UDI has brought uniqueness to the identified product. You can’t deny the impact that will have in the long term,” Kevin Capatch told the audience at a recent AIM-sponsored UDI webinar. Capatch is director of process engineering at Geisinger, a large healthcare system that is a leader in the provider segment in using UDI. “But we haven’t done as well in having the UDI be the single source of the truth.” Reed’s organization has identified over 800 hospitals and other healthcare facilities using UDI. For perspective, there are 6,129 hospitals in the U.S. in 2023, according to the American Hospital Association (AHA). The Engine is Built; Who Will Drive? Today the foundation for multiple UDI use cases and benefits has been set. The FDA set a phased implementation schedule for manufacturers to create and register unique device identifiers for most non-Class 1 medical devices, and the final mandatory participation deadline was December 2022. As of August 2023, there were 4.15 million unique device records in the GUDID database. The database first surpassed 1 million entries in September 2016, reached 2 million in 2018, 3 million in 2021 and 4 million earlier in 2023. In June 2023, there were 2,367 file downloads from the online database, which averaged 6,221 user sessions per day, about a third of which came from outside the U.S. In 2015, the U.S. Department of Health and Human Services issued a rule requiring electronic health record (EHR) systems (e.g., Allscripts, Cerner, Epic and others) to support UDI in their software for it to be certified. Besides requiring medical devices to be uniquely identified and registered in the GUDID, the FDA requires a GUDID reference on other data submitted to the agency. The FDA reported that 89 percent of the device recall notices it received in Q3 2023 included UDI data, double the level from Q1 2022. Figure 1: Number of Devices Recorded in the FDA Global Unique Device Identification Database (GUDID) Source: U.S. Food & Drug Administration There is clearly a lot of data being accessed. The problem is UDI data is often being used alongside many legacy data formats and dependent processes. What was intended as the single source of the truth is currently being used in parallel systems because manufacturers, distributors, hospitals and other stakeholders haven’t changed their data systems and related processes and applications to run on UDI data.
“When legacy IT systems are working, it is very hard to get budget to upgrade them,” notes Konduri. “UDI needs a champion within organizations.” Crowley says most device makers covered by the UDI regulation met their compliance requirements and have done little since, putting them at risk of falling out of compliance. He says there is a small, top tier of leading companies that use UDI extensively in their internal operations, have ongoing engagement with the FDA and supply chain partners and continue to pioneer new use cases. In between, he estimates a tier of 200 – 300 companies that are keeping up with requirements but are not actively advancing use. The entities that have had to put the most into the UDI program – device makers and the FDA – each want to see it used for more than basic compliance. They have invested a lot to create the system and want it to produce more benefits in patient safety, hospital reimbursement, supply chain efficiency and security and clinical research. There is a sense that many stakeholders are waiting for others to make the next move. Until something happens (for device makers, UDI marking and registration were mandatory, not voluntary), many organizations will continue to use their own data systems and related processes. “I believe that UDI adoption would be further along if more organizations – manufacturers, IT vendors, healthcare consultants, the FDA, VA, DOD, HIS and registries – shared the goal of working toward making UDI and a core set of data in GUDID the source of truth in transactions,” says Reed. That attitude extends to UDI’s most influential advocate, the FDA. “Going forward, we’d like to see more focus on broadening UDI adoption, especially in the supply chain and in hospitals for post-market surveillance,” says Konduri. UDI advocates are frustrated by the lack of adoption momentum, particularly in the provider sector because many UDI-based use cases have proven their value. For example, the NEST Coordinating Center (cc) Playbook for Health System UDI Implementation at the Point of Care that was published in 2023 documents different ways providers can benefit from UDI and references real-world examples. Every source interviewed for this article could cite numerous successful programs. What’s Next? Increased adoption is expected to take three forms: incremental growth from medical device makers and distributors as they rationalize their systems and replace NHRC, NDC and proprietary number systems used on products, packaging and internal processes; internationally, because the UDI program has spawned similar efforts in the EU and more than a dozen other countries; and the U.S. healthcare sector, where hospital utilization is seen as the Holy Grail for UDI to produce patient safety benefits. AIM and the American Hospital Association’s Association for Health Care Resource & Materials Management (AHRMM) are currently among the organizations that are most actively promoting UDI adoption by educating potential users. AHRMM scheduled its 2023 UDI Forum for approximately one month after AIM held its own. AIM is active in AHRMM’s UDI Learning Community, which has produced many resources to help hospital professionals to use the UDI system and see its value. Many professionals in the UDI community believe incremental adoption will continue but there won’t be a significant update without a new mandate. The mandate is not likely to come from the FDA, which has fulfilled its mission of creating the UDI system. Hopes for a regulatory catalyst suffered a setback this summer when the National Committee on Vital and Health (NCVH) Statistics recommended against requiring including the Data Identifier (DI) segment of the UDI (the UDI-DI) on the standard 837 electronic claims forms for insurance claims for procedures involving implants. Doing so would help ensure that specific devices are associated with specific patients. That would be an integral step to improving recalls, making data available for robust postmarket surveillance to support patient safety and could help streamline reimbursement operations. A similar, voluntary post-surveillance program for breast implants (the National Breast Implant Registry) grew to include more than 1,500 participating surgeons and over 92,000 records in approximately its first five years. Natalia Wilson, MD, MPH, called the NCVH claims form decision “very disappointing.” Wilson is executive director of the Center for Healthcare Delivery and Policy at Arizona State University and coauthored the NESTcc UDI implementation playbook for health systems. The requirement for including the UDI on claims forms is considered stalled, not dead, because it has many supporters. “If that were to go forward, I think that will be a huge driver for UDI adoption,” says Konduri of the FDA. “We’re confident it will go through. The issue is when.” Several weeks before NCVH opted not to recommend supporting UDI on claims forms, the American Medical Association published an editorial that endorsed the proposal and touted the benefits it could produce. Here is an excerpt from the Journal of the American Medical Association (JAMA) article: “Surveillance of all medical devices, including those modified through PMA supplements, would become feasible, enabling safety concerns to be prospectively identified. More timely notification could also be provided to patients who had been treated with a recalled device, thereby mitigating harm….American healthcare is built to facilitate transactions, and the system’s current transaction standards don’t enable tracking of UDIs. This means that the over 3.5 million devices with UDIs today are functionally invisible to the patients that use them, the providers that purchase them, and the payers that reimburse for them.” “The retail industry is customer experience driven. Our industry tends to be regulation driven,” says Geisinger’s Capatch. “They’re going to be able to do recalls a lot faster than we’re able to do recalls, and that’s just not right.” So, absent an imminent implementation deadline or other regulatory catalyst, expect continued UDI expansion in fits and starts. Many UDI professionals are not satisfied with the current situation, but they don’t think it is permanent either. “I feel we’re closer to getting there, but we’re not getting there,” says Wilson. “I think we’re going to overcome the inertia,” says Capatch of Geisinger. “There is so much opportunity, that is what continues to drive us.”
AIM Welcomes Newly Elected Board Members
March 14, 2023 – Pittsburgh, PA – AIM, the leading industry association and global authority for innovation, standards, and solutions in barcode, biometrics, IoT, NFC, RFID, RTLS, and RAIN technologies, today announces the election results for new members to the Board of Directors. These new board members bring proven leadership abilities and valuable experience to the board with their broad set of experience, skills, diversity, and perspectives. Their term begins March 15, 2023. Leigh Dow, Vice President Global Marketing, Identiv. Leigh is a change agent focused on what’s next, seeking out new ideas, and leading efforts to accelerate growth, build new business and increase brand value. She was recently honored with 2023 Cybersecurity Excellence Awards Marketer of the Year and Podcast of the Year awards. In 2022, she was honored as an inductee to the inaugural Security Industry Association Power 100 and in 2021 named one of The Software Report's Top Women Leaders in Cybersecurity. Leigh is the current Marketing Chair for the UnitVisID Alliance and the Secretary of the NFC Forum IoT SIG. She holds an MBA specializing in Technology Management and a BS in Geopolitical Strategy. Elizabeth Sinclair connects people, industry, academia, standards and technology in her role as Director of Strategic Alliances for Seagull Scientific, developer of BarTender® software. She works with end users and partners to enable consumer confidence, supply chain efficiencies, and regulatory compliance through the deployment of AIDC in industries including aerospace, food, pharma, medical devices, and chemical manufacturing. She has led two AIM committees, chairing both the Track and Trace and Cannabis Traceability work groups. In 2022, Elizabeth was awarded the AIM Professional of the Year. Prior to Seagull Scientific, Elizabeth managed marketing communications for the global chemical distributor Univar, supporting business units in the regulated industries. She attended the University of Washington, and holds a certificate in strategic marketing management from the school’s Foster School of Business. Steven Keddie, AIDC Senior Director for GS1 Global Office. Steven is an Electrical Engineer with 22 years in automotive working for both tier one and OEM (6 automotive patents) and 12 years in marking and coding (focused on FMCG manufacturing). He is an industry expert on printing technology with two marking and coding patents. Steven is a 10-year member of the AIM TSC and author of “Is the GS1-128 Barcode on Course to Become the Preferred Standard for Retail Grocery and Foodservice Packaging?” and leads a global migration to 2D barcode initiative that will see 2D pervasively use in retail by 2027. He is a contributor to several SC31 work efforts and will be launching a new barcode resource for industry to ensure compliance with all GS1’s barcode syntaxes. Steven holds numerous patents and earned a Bachelor of Engineering degree from the Ryerson Polytechnic Institute. “We are excited to expand our board with three new members who will bring diverse expertise and insight to our work,” said Mary Lou Bosco, CEO, AIM, Inc. “We have worked diligently to ensure AIM’s board and leadership represents a variety of experts from industry, and the newest board members are no exception.” For more information, biography, or photos, contact AIM’s headquarters by email or call +1.724.742.4470. Developers of all skill levels recognized for innovative apps that help solve real-world issues impacting businesses.
February 9, 2023 – Pittsburgh, PA – Taking place the 4th and 5th of February, the AIM North America Make-IT-Wright Hackathon hosted nearly 100 students at Wright State University to create solutions to some of the most pressing supply chain challenges in the food and pharmaceutical industries. The students transformed an idea into a business solution in 24 hours. The event featured three training sessions on automatic identification and data capture technologies, standards, labeling design, hardware, software, GS1 Digital Link, EPCIS, plus innovation coaching, mentoring and networking with dedicated industry experts from Avery Dennison, GS1 US, BlueStar, NOVEXX Solutions, BarTender, TSC Printronix, Mojix, and AIS. The hackathon kicked off with an inspirational message from Frank Yiannas, Deputy Commissioner, Food Policy & Response, U.S. FDA along with a visit from U.S. Senator Sherrod Brown’s Legislative Staff member, Pancho Rivera. “The teams participating in this event were very driven to achieve their visions,” shared Jeanne Duckett from Avery Dennison, lead hackathon designer and AIM North America chair, “The results were impressive and provide an excellent example of what is possible when people with the right mix of skills come together and work hard toward a common goal.” A panel of judges from industry and academia evaluated submissions based on level of innovation, real-world impact, and execution. Entries ranged from freshman to graduate students. Award winners shared $6,000 in prizes. The winners included: First Place: Ean Hatfield, Ryan Miller, Braden Pennie, and Kalen Tullis Second Place: Joseph Sodergren, Derrick Thompson, Ethan Thompson, and Liam Williams-Henninger Third Place: Mason McDaniel, Nicole Pililyan, Regin Potter, and Anmol Saini “We congratulate the winning teams for their hard work and innovation, as well as everyone who submitted entries and took the opportunity to develop new skills,” add Matt Kijowski, Cyber Systems Program Manager at Wright State University. “The event showcases the importance of innovation to our economy and our everyday life. The pandemic adds urgency to that message, as innovation will be a key driver of economic recovery,” said Mary Lou Bosco, CEO, AIM North America. The Make-IT-Wright hackathon was organized by AIM North America and Wright State University and partners from key organizations including Avery Dennison, MHEFI, AIDC100, GS1 US, Honeywell, BlueStar, Zebra Technologies, NOVEXX Solutions, BarTender, TSC Printronix, Mojix, AIS, CDO Technologies, and Aware Innovations. For more information or photos, contact AIM NA's headquarters by email or call +1.724.742.4473.
December 12, 2022 – Pittsburgh, PA – AIM North America (AIM NA) has selected winners for their annual awards, including ‘Organization of the Year’, ‘AIT in Government’ and ‘Professional of the Year’ (this year there was a two-way tie). AIM NA extends congratulations to all those who were nominated for these recognitions and honors those who won each category.
“We applaud the award winners on their vision and passion that continue to drive technology innovations and AIDC awareness and implementation,” said Mary Lou Bosco, CEO of AIM North America. AIT In Government Award Since 2013, this award is presented each year to an individual, committee, group, or organization from the government sector that have championed the successful use of AIT within the local, state, or national government. This award is sponsored by the Public Policy Committee and this year’s recognition goes to U.S. Senator Sherrod Brown. U.S. Senator Sherrod Brown serves on the Senate Agriculture Committee, leading the way on how best to secure and shorten food supply chains and other priorities for Ohio farmers in the 2023 Farm Bill. A key consideration in the Farm Bill is providing funding allocations for technology on farms to enable traceability. Other efforts to increase AIDC support in Ohio include the CHIPS Act which lays down a new marker: the technology of the future – from semiconductors to batteries to electric vehicles – will be developed in America and made in America. Lastly, Senator Brown sponsored the development of youth (grades 6-8) Summer Manufacturing Camps and in 2022, the students participated in tours of various manufacturing facilities, including the 9th largest egg production plant in the country which has implemented end to end traceability. Organization of the Year Award Established in 2013, this award is presented to an organization in recognition of outstanding contributions and service that have furthered the growth of the industry. As a respected and responsible leader in the automatic identification and data capture industry, this company’s achievements in serving the industry deserve special recognition. This year’s winner is Metalcraft. Metalcraft announced Inlay Innovation, a wholly owned subsidiary focused on custom RFID inlay design, testing and production. The investment strengthens Metalcraft’s supply chain and meets growing demand for custom products sought by RFID converters, manufacturers, distributors, and integrators. Inlay Innovation offers custom RFID inlay design and manufacturing using state-of-the-art equipment and process, continuing Metalcraft’s 70-year history of technological advancements. Inlay Innovation’s new facility in Ames, Iowa features Mühlbauer Group equipment for production with support from Iowa State University’s Center for Industrial Research and Services. Professional(s) of the Year Award Since 2011, this award is presented annually to an individual in recognition of innovative and exceptional contributions to the development of the Automatic Identification and Mobility industry through their work as a contributor, collaborator, or mentor. This year, the awardees, in a two-way tie, are Elizabeth Sinclair and Susan Flake. Elizabeth Sinclair is the Director, Strategic Alliances for Bartender® by Seagull Scientific and is the Chair of the AIM North America Cannabis Work Group. She has a strong understanding of the emerging cannabis industry needs for automatic identification solutions and guides the Work Group with this vision in mind. Elizabeth was instrumental in leading the Work Group to publish a whitepaper summarizing AIDC technologies and the applications within the cannabis industry. Her knowledge of the cannabis issues/needs in conjunction with her expertise in AIDC are a valuable asset for both AIM and the AIDC community. Susan Flake is the Global Director, Business Development Food and Logistics for Avery Dennison Identification Solutions. She has led the design, deployment, and evolution of almost every major RFID implementation in the retail and food space in North America. She is an expert in RFID reader portfolios, tag to reader behavior and key innovation for RFID software applications specific to the food industry needs of expiry management, traceability, and inventory management. Her wealth of knowledge and her passion have been key to the advances of AIDC within the food industry and the work conducted by the Food Supply Chain Work Group. For more information, biography, or photos, contact AIM NA's headquarters by email or call +1.724.742.4473. Experts share expertise to direct and guide organizations of
asset tracking technologies from cultivation to consumer April 6, 2022 – Pittsburgh, PA – The Cannabis Work Group of AIM North America (AIM NA), the premier alliance for the automated data capture technologies industry, including barcode, RFID, IoT, NFC, and RTLS, released a whitepaper that provides an overview and general recommendations to help guide the effort toward cannabis traceability from cultivation to consumer. The Track and Trace for the Cannabis Industry from Cultivation to Consumer Whitepaper, was written for all members of the cannabis supply chain — farmers and growers, processors, manufacturers, distributors, dispensaries, even consumers — who benefit from implementing AIDC solutions. This whitepaper will
“Growers, processers, and retailers are navigating significant challenges in a relatively new industry. The need to comply with traceability regulation adds a layer of complexity to what it is they do,” stated Elizabeth Sinclair, Cannabis Work Group Chair and Director of Marketing for BarTender® Software. “This whitepaper is a primer on the AIDC technologies that will enable regulatory compliance and process efficiencies at scale as the cannabis industry grows.” The Cannabis Work Group Membership represent all industries and organizations that use, implement, resell, or develop automatic identification and data capture (AIDC) technologies. These technologies are essential to enabling adoption, growth, and interoperability to those who depend on accurate, available, and identifiable data. For additional information and to inquire about membership, please click here. |
Archives
April 2024
Categories |










































 RSS Feed
RSS Feed