|
September 23, 2023, marked the 10-year anniversary of the day the FDA’s Unique Device Identification (UDI) requirement first took effect. For many UDI pioneers and leaders, the date represented a 20-year anniversary since UDI development work began about a decade before UDI became law. In that time, UDI went from an idea to a framework to a law; its GUDID database now uniquely identifies and holds data on more than 4 million medical devices and is the foundation for thousands of daily lookups and transactions. It supports a growing range of use cases in supply chain operations, hospital material management, patient safety and clinical research. UDI set a precedent and serves as a model for similar medical safety and supply chain systems in dozens of countries around the world. The FDA reported that 89 percent of device recall submissions in Q3 2023 included the UDI, double the level from Q1 2022. UDI’s progress is exceptional, considering where it started, and the many diverse stakeholders involved. Yet many professionals that were most responsible for creating and advancing the UDI system are not celebrating these accomplishments but instead are focused on moving the program forward. “Over the last 10 years, a lot of progress has been made,” says Indira Konduri, who is deputy Director of Division of Surveillance Support within the FDA’s Center for Devices and Radiological Health (CDRH), and is leading UDI implementation for the Center. “The good news is that the infrastructure the UDI system needs is in place. Now we’re doing a lot of exciting work leveraging this infrastructure and utilizing UDI to meet the intent of the UDI rule.” AIM NA has supported the UDI program at every step, starting years before UDI became a standard and a regulation. The association provided expert input to every FDA request for comment while the regulations were being developed. Post implementation, AIM provided vendor-neutral technical assistance to help respond to queries the FDA’s UDI help desk. The AIM NA UDI for Medical Devices Work Group remains active in developing resources and sharing knowledge to advance the UDI program and encourages new participants to join. This article presents a snapshot of the current state of the UDI and how the movement may evolve. The Origin Story In the early 2000s, consumer goods were routinely identified with a U.P.C./EAN (now GTIN) number and bar code, and U.S. drug products had the National Drug Code (NDC). There was no standardized equivalent for medical devices. Device makers, safety advocates, regulators and others envisioned a program for medical devices that would go beyond those efforts, which identify items at the category or packaging level, by providing a shareable, unique identification record for every individual device. “The basic reason for the UDI program was to create an NDC system for devices,” says Jay Crowley, who was the original FDA official for the UDI program and now works in the private sector as a UDI consultant and advocate for USDM Life Sciences. He is also the current chair of the AIM NA UDI for Medical Devices Work Group. After extensive consultation among organizations representing various sectors of the life sciences and healthcare sectors, the FDA set the tenets for building the UDI system:
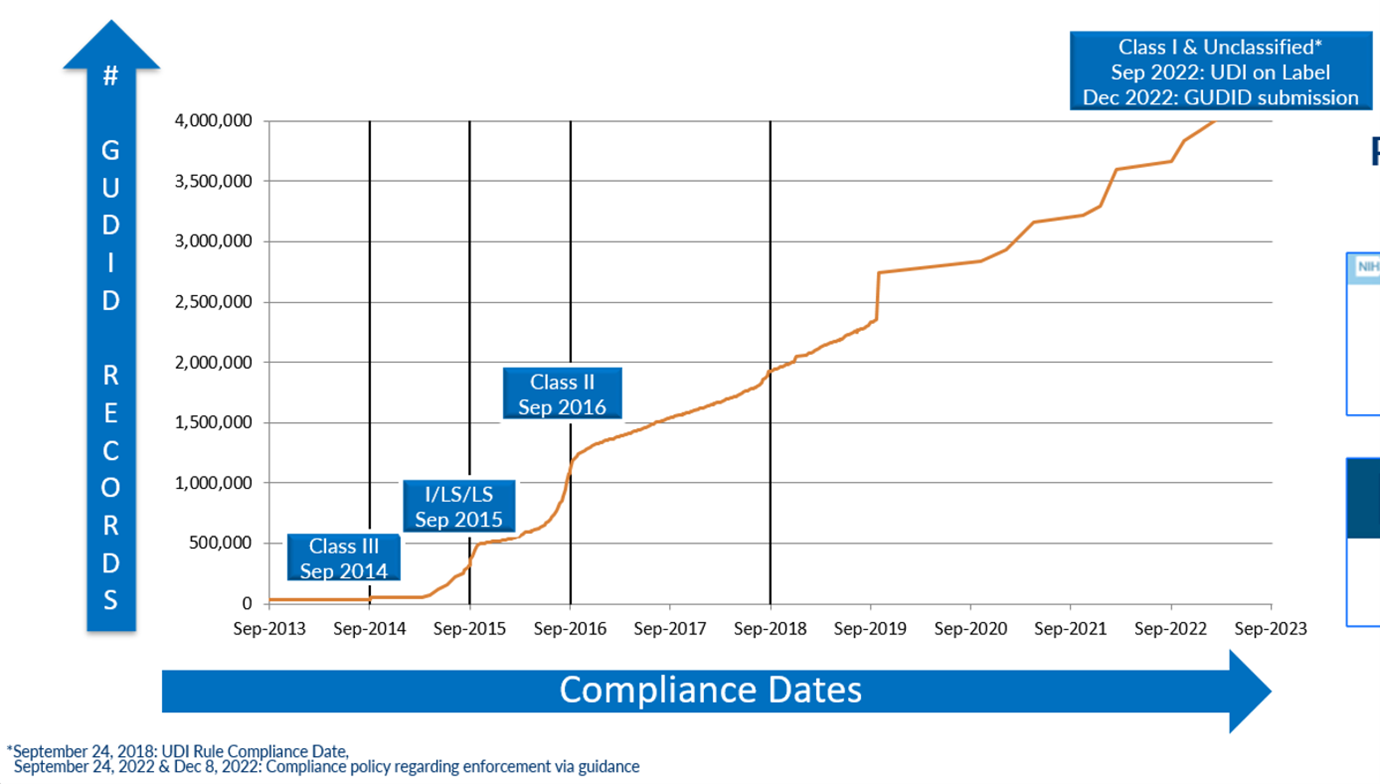
“This new form of device identification, when adopted in health IT systems, could be used as authoritative master data,” says Crowley. “It would increase supply chain efficiency, allow for improved reporting of adverse events, better detection of device safety signals, improved maintenance of biomedical equipment, improved tracking of implants, more efficient and effective recalls, and better monitoring of patient outcomes in clinical care and registry data by replacing unstructured and nonstandard device identification data with a unique number and associated public regulatory data.” To get there, UDI planners had to ask basic questions, starting with, what should be identified? What options should be available for scannable data – could existing standards be used, or was a new one needed? Which database fields should be mandatory and what could be optional? There were hundreds of more detailed questions. Setting the specifications for data formats was especially challenging. A few of the original questions have not been completely answered even as the GUDID database has surpassed 4 million records. There is still some confusion among device makers and others about the minutiae of data formats and their application. Plus, after 10 years, many organizations have lost valuable institutional knowledge because of employee turnover. The biggest remaining question, and frustration, is how to get more organizations using UDI. “We anticipated that moving from ‘chaos’ and nonstandard device to structured identification would take a long time and that, over time, we would uncover and resolve data quality issues,” says Terrie Reed. She is a former FDA official who now helps healthcare organizations take advantage of UDI in their operations in her role as chief strategy officer at Symmetric Health Solutions. “We did not anticipate that each manufacturer would interpret the regulation and its intention differently, and that so much emphasis would be placed on meeting FDA regulatory requirements vs. working with healthcare systems to understand how to implement UDI in a way that would achieve expected patient safety, device safety, supply chain and other commercial benefits.” “UDI has brought uniqueness to the identified product. You can’t deny the impact that will have in the long term,” Kevin Capatch told the audience at a recent AIM-sponsored UDI webinar. Capatch is director of process engineering at Geisinger, a large healthcare system that is a leader in the provider segment in using UDI. “But we haven’t done as well in having the UDI be the single source of the truth.” Reed’s organization has identified over 800 hospitals and other healthcare facilities using UDI. For perspective, there are 6,129 hospitals in the U.S. in 2023, according to the American Hospital Association (AHA). The Engine is Built; Who Will Drive? Today the foundation for multiple UDI use cases and benefits has been set. The FDA set a phased implementation schedule for manufacturers to create and register unique device identifiers for most non-Class 1 medical devices, and the final mandatory participation deadline was December 2022. As of August 2023, there were 4.15 million unique device records in the GUDID database. The database first surpassed 1 million entries in September 2016, reached 2 million in 2018, 3 million in 2021 and 4 million earlier in 2023. In June 2023, there were 2,367 file downloads from the online database, which averaged 6,221 user sessions per day, about a third of which came from outside the U.S. In 2015, the U.S. Department of Health and Human Services issued a rule requiring electronic health record (EHR) systems (e.g., Allscripts, Cerner, Epic and others) to support UDI in their software for it to be certified. Besides requiring medical devices to be uniquely identified and registered in the GUDID, the FDA requires a GUDID reference on other data submitted to the agency. The FDA reported that 89 percent of the device recall notices it received in Q3 2023 included UDI data, double the level from Q1 2022. Figure 1: Number of Devices Recorded in the FDA Global Unique Device Identification Database (GUDID) Source: U.S. Food & Drug Administration There is clearly a lot of data being accessed. The problem is UDI data is often being used alongside many legacy data formats and dependent processes. What was intended as the single source of the truth is currently being used in parallel systems because manufacturers, distributors, hospitals and other stakeholders haven’t changed their data systems and related processes and applications to run on UDI data.
“When legacy IT systems are working, it is very hard to get budget to upgrade them,” notes Konduri. “UDI needs a champion within organizations.” Crowley says most device makers covered by the UDI regulation met their compliance requirements and have done little since, putting them at risk of falling out of compliance. He says there is a small, top tier of leading companies that use UDI extensively in their internal operations, have ongoing engagement with the FDA and supply chain partners and continue to pioneer new use cases. In between, he estimates a tier of 200 – 300 companies that are keeping up with requirements but are not actively advancing use. The entities that have had to put the most into the UDI program – device makers and the FDA – each want to see it used for more than basic compliance. They have invested a lot to create the system and want it to produce more benefits in patient safety, hospital reimbursement, supply chain efficiency and security and clinical research. There is a sense that many stakeholders are waiting for others to make the next move. Until something happens (for device makers, UDI marking and registration were mandatory, not voluntary), many organizations will continue to use their own data systems and related processes. “I believe that UDI adoption would be further along if more organizations – manufacturers, IT vendors, healthcare consultants, the FDA, VA, DOD, HIS and registries – shared the goal of working toward making UDI and a core set of data in GUDID the source of truth in transactions,” says Reed. That attitude extends to UDI’s most influential advocate, the FDA. “Going forward, we’d like to see more focus on broadening UDI adoption, especially in the supply chain and in hospitals for post-market surveillance,” says Konduri. UDI advocates are frustrated by the lack of adoption momentum, particularly in the provider sector because many UDI-based use cases have proven their value. For example, the NEST Coordinating Center (cc) Playbook for Health System UDI Implementation at the Point of Care that was published in 2023 documents different ways providers can benefit from UDI and references real-world examples. Every source interviewed for this article could cite numerous successful programs. What’s Next? Increased adoption is expected to take three forms: incremental growth from medical device makers and distributors as they rationalize their systems and replace NHRC, NDC and proprietary number systems used on products, packaging and internal processes; internationally, because the UDI program has spawned similar efforts in the EU and more than a dozen other countries; and the U.S. healthcare sector, where hospital utilization is seen as the Holy Grail for UDI to produce patient safety benefits. AIM and the American Hospital Association’s Association for Health Care Resource & Materials Management (AHRMM) are currently among the organizations that are most actively promoting UDI adoption by educating potential users. AHRMM scheduled its 2023 UDI Forum for approximately one month after AIM held its own. AIM is active in AHRMM’s UDI Learning Community, which has produced many resources to help hospital professionals to use the UDI system and see its value. Many professionals in the UDI community believe incremental adoption will continue but there won’t be a significant update without a new mandate. The mandate is not likely to come from the FDA, which has fulfilled its mission of creating the UDI system. Hopes for a regulatory catalyst suffered a setback this summer when the National Committee on Vital and Health (NCVH) Statistics recommended against requiring including the Data Identifier (DI) segment of the UDI (the UDI-DI) on the standard 837 electronic claims forms for insurance claims for procedures involving implants. Doing so would help ensure that specific devices are associated with specific patients. That would be an integral step to improving recalls, making data available for robust postmarket surveillance to support patient safety and could help streamline reimbursement operations. A similar, voluntary post-surveillance program for breast implants (the National Breast Implant Registry) grew to include more than 1,500 participating surgeons and over 92,000 records in approximately its first five years. Natalia Wilson, MD, MPH, called the NCVH claims form decision “very disappointing.” Wilson is executive director of the Center for Healthcare Delivery and Policy at Arizona State University and coauthored the NESTcc UDI implementation playbook for health systems. The requirement for including the UDI on claims forms is considered stalled, not dead, because it has many supporters. “If that were to go forward, I think that will be a huge driver for UDI adoption,” says Konduri of the FDA. “We’re confident it will go through. The issue is when.” Several weeks before NCVH opted not to recommend supporting UDI on claims forms, the American Medical Association published an editorial that endorsed the proposal and touted the benefits it could produce. Here is an excerpt from the Journal of the American Medical Association (JAMA) article: “Surveillance of all medical devices, including those modified through PMA supplements, would become feasible, enabling safety concerns to be prospectively identified. More timely notification could also be provided to patients who had been treated with a recalled device, thereby mitigating harm….American healthcare is built to facilitate transactions, and the system’s current transaction standards don’t enable tracking of UDIs. This means that the over 3.5 million devices with UDIs today are functionally invisible to the patients that use them, the providers that purchase them, and the payers that reimburse for them.” “The retail industry is customer experience driven. Our industry tends to be regulation driven,” says Geisinger’s Capatch. “They’re going to be able to do recalls a lot faster than we’re able to do recalls, and that’s just not right.” So, absent an imminent implementation deadline or other regulatory catalyst, expect continued UDI expansion in fits and starts. Many UDI professionals are not satisfied with the current situation, but they don’t think it is permanent either. “I feel we’re closer to getting there, but we’re not getting there,” says Wilson. “I think we’re going to overcome the inertia,” says Capatch of Geisinger. “There is so much opportunity, that is what continues to drive us.”
|
Archives
June 2024
Categories |














 RSS Feed
RSS Feed